nivek
As Above So Below
Sunlight could solve Drinking Water's Biggest Problem in 30 Minutes
CLEAN DRINKING WATER is one of life's essentials. However, safe drinking water isn't a reality for over 2 billion people across the world. These individuals are at risk of contracting potentially fatal waterborne illnesses like typhoid and polio.
This at-risk population is expected to grow over the next five-years as the climate crisis creates more and more water-stressed areas, according to the World Health Organization (WHO.)
Scientists have been working to solve this problem for decades, and while some solutions have found success in effectively cleaning water, those energy-heavy solutions can be hard to implement in communities without a stable electric grid.
Now, a team of scientists from Australia and China has proposed a sustainable solution that relies on sunlight to jump-start the filtration process instead of heat or electricity.
Using a super porous material to suck up salt from brackish, salty water, researchers were able to sustainably create nearly 40 gallons of clean drinking water per single kilogram of a metal material. Better yet, this drinking water was even cleaner than WHO's official guidelines.
This finding was published Monday in the journal Nature Sustainability.
The study's lead author Huanting Wang, a professor of chemical engineering at Monash University in Australia, says that his team's approach makes use of the planet's most abundant resource: sunlight. Their solar-powered method desalinates brackish, or stagnate, water more sustainable than previous methods.
"[T]hermal desalination processes by evaporation are energy-intensive, and other technologies, such as reverse osmosis, has a number of drawbacks, including high energy consumption and chemical usage in membrane cleaning and dechlorination," Wang says. "Sunlight is the most abundant and renewable source of energy on Earth."
Wang and his colleagues explain in the study that a sustainable energy source, like sunlight, would be especially useful for communities that may not have access to a reliable electric grid necessary for other methods of desalination.
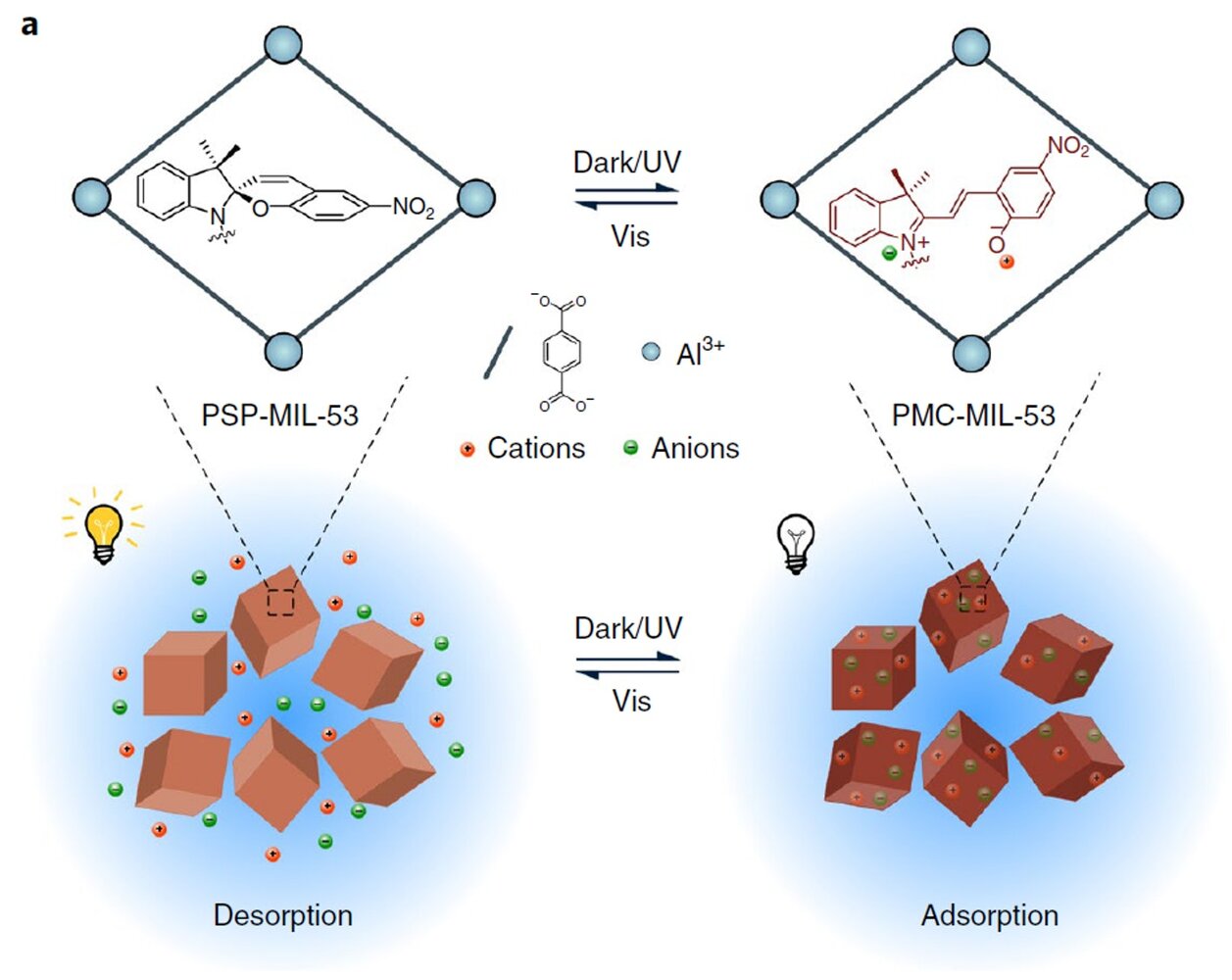
HOW DOES IT WORK — While sunlight is an important part of this process, another key player is the material the researchers chose to use. This material is a type of metal compound that is composed of metal ions configured into a crystalline pattern — not unlike the salt it aims to absorb.
Because of its unique crystalline structure, this compound is incredibly porous, with so many nooks and crevices within it that its overall surface area is actually the largest per unit measure of any known material.
So large in fact, that scientists estimate the entire area of a football field could fit within a single teaspoon of this material. A characteristic that makes it really effective at sucking up salt from water.
The researchers further enhanced the absorption of this material by adding another material to its pores, called PSP-MIL-53. This material is characterized by having "breathing effects," and is able to promote efficient absorption.
After testing this material on both natural saltwater and synthetic saltwater, they found that the compound was able to absorb enough water in 30 minutes to create nearly 40 gallons of fresh drinking water per single kilogram of the material.
When analyzing the resultant water, the researchers measured its total dissolved solids (TDS) to be less than 500 parts per million — a standard even above that recommended by WHO, which categorizes clean drinking water as having TDS no greater than 600 parts per million.
The initial absorption is done in the dark but a four-minute exposure to sunlight causes the material to release its collected salt and begin the absorption process again for many more cycles.
"This study has successfully demonstrated that the photoresponsive [metal compounds] are a promising, energy-efficient, and sustainable adsorbent for desalination," said Wang. "Our work provides an exciting new route for the design of functional materials for using solar energy to reduce the energy demand and improve the sustainability of water desalination."
WHAT'S NEXT — In addition to helping provide a sustainable solution to creating clean drinking water for communities with poor energy infrastructure, the researchers also say that this approach could be repurposed in the future for the absorption of other compounds and minerals, creating a sustainable solution for mineral mining as well. What has to happen next is determining how to get this tech out of the lab, and into the field.
.
CLEAN DRINKING WATER is one of life's essentials. However, safe drinking water isn't a reality for over 2 billion people across the world. These individuals are at risk of contracting potentially fatal waterborne illnesses like typhoid and polio.
This at-risk population is expected to grow over the next five-years as the climate crisis creates more and more water-stressed areas, according to the World Health Organization (WHO.)
Scientists have been working to solve this problem for decades, and while some solutions have found success in effectively cleaning water, those energy-heavy solutions can be hard to implement in communities without a stable electric grid.
Now, a team of scientists from Australia and China has proposed a sustainable solution that relies on sunlight to jump-start the filtration process instead of heat or electricity.
Using a super porous material to suck up salt from brackish, salty water, researchers were able to sustainably create nearly 40 gallons of clean drinking water per single kilogram of a metal material. Better yet, this drinking water was even cleaner than WHO's official guidelines.
This finding was published Monday in the journal Nature Sustainability.
The study's lead author Huanting Wang, a professor of chemical engineering at Monash University in Australia, says that his team's approach makes use of the planet's most abundant resource: sunlight. Their solar-powered method desalinates brackish, or stagnate, water more sustainable than previous methods.
"[T]hermal desalination processes by evaporation are energy-intensive, and other technologies, such as reverse osmosis, has a number of drawbacks, including high energy consumption and chemical usage in membrane cleaning and dechlorination," Wang says. "Sunlight is the most abundant and renewable source of energy on Earth."
Wang and his colleagues explain in the study that a sustainable energy source, like sunlight, would be especially useful for communities that may not have access to a reliable electric grid necessary for other methods of desalination.

HOW DOES IT WORK — While sunlight is an important part of this process, another key player is the material the researchers chose to use. This material is a type of metal compound that is composed of metal ions configured into a crystalline pattern — not unlike the salt it aims to absorb.
Because of its unique crystalline structure, this compound is incredibly porous, with so many nooks and crevices within it that its overall surface area is actually the largest per unit measure of any known material.
So large in fact, that scientists estimate the entire area of a football field could fit within a single teaspoon of this material. A characteristic that makes it really effective at sucking up salt from water.
The researchers further enhanced the absorption of this material by adding another material to its pores, called PSP-MIL-53. This material is characterized by having "breathing effects," and is able to promote efficient absorption.
After testing this material on both natural saltwater and synthetic saltwater, they found that the compound was able to absorb enough water in 30 minutes to create nearly 40 gallons of fresh drinking water per single kilogram of the material.
When analyzing the resultant water, the researchers measured its total dissolved solids (TDS) to be less than 500 parts per million — a standard even above that recommended by WHO, which categorizes clean drinking water as having TDS no greater than 600 parts per million.
The initial absorption is done in the dark but a four-minute exposure to sunlight causes the material to release its collected salt and begin the absorption process again for many more cycles.
"This study has successfully demonstrated that the photoresponsive [metal compounds] are a promising, energy-efficient, and sustainable adsorbent for desalination," said Wang. "Our work provides an exciting new route for the design of functional materials for using solar energy to reduce the energy demand and improve the sustainability of water desalination."
WHAT'S NEXT — In addition to helping provide a sustainable solution to creating clean drinking water for communities with poor energy infrastructure, the researchers also say that this approach could be repurposed in the future for the absorption of other compounds and minerals, creating a sustainable solution for mineral mining as well. What has to happen next is determining how to get this tech out of the lab, and into the field.
Abstract: Light-responsive materials with high adsorption capacity and sunlight-triggered regenerability are highly desired for their low-cost and environmentally friendly industrial separation processes. Here we report a poly(spiropyran acrylate) (PSP) functionalized metal–organic framework (MOF) as a sunlight-regenerable ion adsorbent for sustainable water desalination. Under dark conditions, the zwitterionic isomer quickly adsorbs multiple cations and anions from water within 30 minutes, with high ion adsorption loadings of up to 2.88 mmol g−1 of NaCl. With sunlight illumination, the neutral isomer rapidly releases these adsorbed salts within 4 minutes. Single-column desalination experiments demonstrated that PSP–MOF works efficiently for water desalination. A freshwater yield of 139.5 l kg−1 d−1 and a low energy consumption of 0.11 Wh l−1 would be reached for desalinating 2,233 ppm synthetic brackish water. Importantly, this adsorbent shows excellent stability and cycling performance. This work opens up a new direction for designing stimuli-responsive materials for energy-efficient and sustainable desalination and water purification.
.
